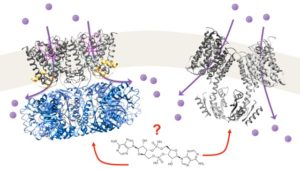
Potassium homeostasis is essential for bacterial survival and controlled by the orchestrated function of various K+ importers and exporters. Recently, cyclic di-AMP has been identified as overarching regulatory signaling molecule for K+ homeostasis in different Gram-positive bacteria. It regulates the uptake and release of potassium by controlling gene expression and protein activity. In Bacillus subtilis, cyclic di-AMP directly binds to K+ importers KtrAB and KimA leading to their inhibition.
We aim to shed light on the underlying mechanisms of protein deactivation elucidating the molecular principles of cyclic di-AMP binding and transport inhibition.